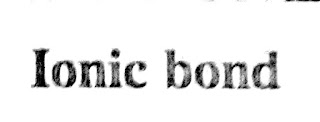Friday, 28 December 2018
Thursday, 27 December 2018
Different Scales Of Temperature
➡The temperature of an object or body is measured in 4 different scales.
1. Celsius or centigrade scale
2. Fahrenheit scale
3. Kelvin scale
4. Reaumur scale
1. Celsius or centigrade scale:
➡Anders Celsius devised the scale in 1710.
➡Lower fixed point of Celsius scale is 0 degree celsius.( as Ice melts are freezes at 0 degree Celsius)
➡Upper fixed point of Celsius scale is 100 degree Celsius.( water starts boiling at 100 degree Celsius)
➡Fundamental interval is divided into 100 equal divisions all parts.
➡Each division gives a temperature of 1 Degree Celsius.
➡Each Degree on celsius scale is 1/100 part of interval between the upper fixed point and lower fixed point.
2. Fahrenheit scale:
➡Gabriel Fahrenheit devised the fahrenheit scale in 1717.
➡Lower fixed point of fahrenheit scale is 32° F.
➡Upper fixed point of fahrenheit scale is 212° F.
➡Fundamental interval in fahrenheit scale is divided into 180 equal parts or divisions.
➡Each degree on fahrenheit scale is divided into 1/ 180 th part of difference between the lower fixed point and upper fixed point.
3. Kelvin scale:
➡Lord Kelvin devised this Kelvin scale.
➡Lower fixed point of Kelvin scale is 273 K.
➡Upper fixed point of Kelvin scale is 373 K.
➡Fundamental interval in kelvin scale is divided into 100 equal parts or divisions.
➡Each degree on Kelvin scale is 1/100 part of interval between the upper fixed point and lower fixed point.
➡0 K is equal to - 273° C, which is known as absolute zero of temperature. It is the lowest temperature attained by any body.
4. Reaumur scale:
➡Reaumur devised this reaumur scale in 1730.
➡Lower fixed point of Reaumur scale is 0° R.
➡Upper Fixed point of Reaumur scale is 80° R.
➡Fundamental interval in Reaumur scale is divided into 80 equal parts or divisions.
➡Each degree on Reaumur scale is 1 by 80 part of lower fixed point and upper fixed point.
Wednesday, 26 December 2018
Transmission Of Heat

"Heat energy always flows from a body of high temperature to a body of low temperature."
➡This flow of heat energy between the bodies takes place in 3 different process:
1. Conduction
2. Convection
3. Radiation
➡Flow of heat energy between the bodies takes place until it reaches the thermal equilibrium.
1. Conduction
➡Conduction a process in which heat energy flows from one molecule to another molecule without the actual movement of the particles.
➡Here the molecules upon attaining heat energy start vibrating at greater amplitude without change in its position and transfers the energy gained into the nearby molecules.
➡Solid mainly undergo the process of conduction.
➡Example: Heating of one end of an iron rod, there by transfer of heat energy between the molecules takes place without the movement of the molecules,the other end of the iron rod which is cold, becomes hot.
2. Convection
➡Convection is a process in which transfer of heat energy takes place between one molecule to another molecule by the actual movement of the molecules.
➡Transfer of heat energy is always vertically upwards in the process of convection.
➡The molecules which is near to the surface becomes Less dense on absorbing heat energy, so it Rises up which is called a convection current.
➡Liquids and gases are mainly goes under the process of convection.
➡The convection current continues until the entire liquid or gases aquires the same temperature.
➡Heat energy through Convection is not done in vacuum.
3. Radiation
➡Radiation is the process in which heat energy directly passes from one body to another body without the involvement of the medium.
➡Heat transfer through the process of radiation is also takes place in vacuum.
➡Transfer of heat energy by the process of radiation is called Radiant heat or the thermal radiation.
➡Radiant Heat travels in all directions.
➡Radiant Heat travels in a straight lines with the velocity of light and can also passes through vacuum.
➡Radiant heat obey the laws of reflection.
➡The material through which radiant Heat passes do not heat the material.
Tuesday, 25 December 2018
Humidity And Relative Humidity
"The amount of water vapour present in air at a given temperature is known as Humidity."
➡The amount of water vapour in air changes with change in temperature.
➡Maximum amount of water vapour contained in the air at particular temperature is called as Saturated air of the temperature.
➡Higher the temperature, more is the capacity of the air to hold water vapour.
➡If the temperature of agitated air is increased then it becomes unsaturated and if the temperature of the air is decreased then it is saturated with condensation of water vapour.
➡Dew is formed during night due to condensation of excess water vapour with fall of temperature.
Relative Humidity:
"It is defined as the ratio of mass of water vapour present in 1 cubic metre of air at a certain temperature to the mass of water vapour required to saturate 1 cubic metre of air temperature known as relative humidity."
➡It is expressed in percentage.
Monday, 24 December 2018
Physical Quantity And System Of Units
"Any quantity that are measured in physics are called as physical quantity."
➡Physical quantity measurement required a magnitude and an unit.
➡Physical quantity measurement= magnitude (N) X unit (U)
➡Here Magnitude tells about number of times the unit is present in physical quantity
and Unit tells about a standard that is used to measure a physical quantity.
Types of physical quantity:
1. Fundamental quantity
2. Derived quantity
1. Fundamental quantity
➡Physical quantity which is independent of any other quantities is called as
fundamental quantity.
➡Fundamental quantity is also called as Basic quantity
Example: Length, mass, time
2. Derived quantity
➡A physical quantity which can be obtained from fundamental quantity is called a
derived quantity.
Example: Area, speed, etc
System of units:
➡To measure Physical quantities 3 Types of System of units are being used,
1. F.P.S system
➡Unit of length is foot
➡Unit of mass is pound
➡Unit of time is second
2. C.G.S system
➡Unit of length is centimetre
➡Unit of mass is gram
➡Unit of time is second
3. M.K.S/ S.I system
➡Unit of length is metre
➡Unit of mass is kilogram
➡Unit of time is second
Sunday, 23 December 2018
Latent Heat
"The amount of heat required to change its state of any substance without
changing its temperature is called latent heat."
As we know,
➡Quantity of heat is directly proportional to mass of substance.
Q ∝ m
Q = m L
L = Q/m
➡The SI unit of latent heat is joules per kg
Types of latent Heat:
➡Latent heat is of two types
1. Latent heat of fusion
2. Latent heat of vaporization
➡1. Latent heat of fusion:
"The amount of heat required to convert unit mass of a substance from solid to
liquid without change in temperature is called latent heat of fusion."
➡Latent heat of fusion of ice is 80 calories per gram.
➡2. Latent heat of vaporization:
"The amount of heat required to convert unit mass of substance from liquid to
Vapour without changing its temperature is called latent heat of vaporization."
➡Latent heat of vaporization of water is 540 calories per gram.
Saturday, 22 December 2018
Specific Heat

"The amount of heat required to raise the temperature of unit mass of a substance by 1 unit is known as specific heat of a substance."
➡When two bodies at different temperatures are brought in contact with each other, the heat energy flows from body of high temperature to the body of low temperature till the temperature becomes equal.
The factors upon which the quantity of heat depends are:
➡The quantity of heat depends on the mass of a body.
Q ∝ m
➡Quantity of heat depends on change in temperature of body.
Q ∝ (T2 - T1)
➡Quantity of heat depends on the nature of material.
Q ∝ m.(T2 - T1)
Q = m. C. (T2 - T1)
➡Here heat absorbed or give out is equals to mass X specific heat X change in
temperature.
C = Q/ (T2 - T1)
➡Here C is called the specific heat of the body which depends on the nature of
material of body.
➡Among all substances, water has the highest specific heat capacity.
Friday, 21 December 2018
Fundamental Unit And Rules For Writing Symbols Of Unit
"The unit which are used for measuring fundamental quantities are called as fundamental units."
➡Fundamental quantity is nothing but any physical quantity which is independent of any other quantity is called fundamental quantity.
Example- length, mass, time, etc.
➡Fundamental unit is also called as Basic Unit.
➡Length, mass, time are called the basic unit or fundamental unit which are together called as the System of units.
Example: length - metre, mass - kilogram, time - second
Rules for writing symbols of units:
➡1. The unit named after a scientist must be written in small letters when the complete name is written.
Example: Force unit should be written in newton not in Newton.
➡2. When only the first letter of the scientist name is used as a symbol then it should written in capital letter.
Example: Force unit should be written in J not j.
➡3. Other that scientist names, all the units are represented only by small letters.
➡4. Symbols for Units should only be written in singular form.
➡5. No full stop or punctuation marks should be written at the end of simple units.
Example: Mass of a body is 25 kg but not 25 kg.
Thursday, 20 December 2018
Ionic bond
"Transfer of electrons from the metal atoms to the non metal atom forming a positive ions and negative ions which gets attracted due to electrostatic forces forms a chemical bond called as ionic bond."
➡Ionic bond was proposed by Kossel.
➡Since bond is formed due to electrostatic force, ionic bond is also called as electrostatic Bond.
➡Transfer of electrons from atom of one element to the atom of other element takes place in ionic bond.
➡Highly reactive metals like alkali metals of group 1A and highly reactive nonmetal like halogens of group 7A usually participate in ionic bond.
➡Ionic bond is formed between atoms of elements with electronegativity difference equal to or greater than 1.9
➡Here in ionic bond formation either atom will lose electrons or the atom will gain electrons to attain octet in valence shell.
➡This tendency of losing electrons to form cations or gaining electrons to form anions depend on the following factors:
1. Atomic size
2. Ionisation potential
3. Electron affinity
4. Electronegativity
➡Atoms of elements with high ionization potential, high electron affinity ,small atomic size , high electronegativity form anions.
➡Elements with low ionization energy, low electron affinity, high atomic size and low electronegativity form cations.
Wednesday, 19 December 2018
Hydrocarbons
"Compounds which contains only carbon and hydrogen in their molecules are called hydrocarbons."
Types of hydrocarbons:
Hydrocarbon are classified into two types.
1. Aliphatic hydrocarbons/ Acyclic hydrocarbons.
➡ Aliphatic hydrocarbons are also called open chain hydrocarbons.
➡ Again classified into two types:
a. Saturated hydrocarbons.
➡ saturated means chemically unreactive.
➡ alkanes comes under saturated hydrocarbons.
➡Hydrocarbons which contains only single bonds between carbon atoms are called alkanes.(c-c)
➡General formula for alkanes:
CnH2n+2
b. Unsaturated hydrocarbons.
➡ alkenes and alkynes comes under unsaturated hydrocarbons.
➡ hydrocarbons which contains at least one double bond between carbon atoms are called alkenes.(c=c)
➡ general formula for alkenes:
CnH2n
➡ hydrocarbons which contains at least one triple bond between carbon atoms are called Alkynes.
➡ general formula for alkyne's:
CnH2n-2.
2. Cyclic hydrocarbons/ open chain hydrocarbons
➡ cycloalkanes comes under cyclic hydrocarbons.
Tuesday, 18 December 2018
Image Characteristics
1. When object is at infinity:
➡Image is formed at principal focus.
➡Images highly diminished ,real and inverted.
2. When object placed along the axis:
➡Image formed is vertical and inverted.
➡Images formed between focal point and centre.
➡Image formed is diminished,real and inverted.
3. When object is at the centre of curvature:
➡Image formed at centre of curvature.
➡Image size is same as that of object and it is real and inverted.
4. When object is between centre of curvature and focus:
➡Image is formed beyond C.
➡Image formed is real, inverted and enlarged.
5. When object is at principal focus:
➡Image is formed at infinity.
➡Images of highly enlarged, real and inverted.
6. When object is between principal focus and pole:
➡Images formed behind the mirror.
➡Images of highly enlarged, virtual and erect.
➡Image is formed at principal focus.
➡Images highly diminished ,real and inverted.
2. When object placed along the axis:
➡Image formed is vertical and inverted.
➡Images formed between focal point and centre.
➡Image formed is diminished,real and inverted.
3. When object is at the centre of curvature:
➡Image formed at centre of curvature.
➡Image size is same as that of object and it is real and inverted.
4. When object is between centre of curvature and focus:
➡Image is formed beyond C.
➡Image formed is real, inverted and enlarged.
5. When object is at principal focus:
➡Image is formed at infinity.
➡Images of highly enlarged, real and inverted.
6. When object is between principal focus and pole:
➡Images formed behind the mirror.
➡Images of highly enlarged, virtual and erect.
Monday, 17 December 2018
Power

Power:
Rate of doing work or work done per unit time is called power of the machine or man.
It is a scalar quantity represented by a symbol P.
Power =work / time.
SI unit of power is watt.
1 watt is equals to 1 joules per second.
Higher units of power are 1 Kilowatt, 1 megawatt, 1 gigawatt.
Commercially power is measured in Horsepower.
1 horsepower is equal to 746 watt.
Power can be expressed in different way :
Power is equals to force x distance divided by time.
( when moving in body)
( when moving in body)
Power is equals to minus force x distance by time.
( when stopping a body)
( when stopping a body)
Power is equal to force x velocity cos theta
( when pulling a body)
( when pulling a body)
Power is equals to mX gX h/ time
( when lifting a body against gravity)
( when lifting a body against gravity)
Power is equals to Force X velocity
( when moving with velocity
( when moving with velocity
Wednesday, 12 December 2018
Saturday, 8 December 2018
Friday, 7 December 2018
Significant Figures - Rules For Significant Figures Counting
"Significant Figures - tells about the accuracy of measurement by counting the reliable uncertain digits in the given measurement quantity."
Rules:
* All non zero digits are counted as significant.
ex- 12134 - Number of significant figures = 5
* All the zero's on the right side of the non zero digits are not counted as significant.
ex- 854300 - Number of significant figures = 4
* All the zero's on the right side of non zero digits with a decimal in it are counted as significant.
ex- 4.56800 - Number of significant figures = 6
* All the zero's before the non digits with a decimal in it are not counted as significant.
ex- 0.007340 - Number of significant figures = 4
* All the zero's between the non zero digits are counted as significant irrespective of where the decimal point is placed in it.
ex- 19.00067 - Number of significant figures = 7
** If the significant figure is in the order of a x 10 b
Here 10 to the power's are ignored ( not taken into account), only number of significant figures in a are counted.
ex - 3.8 x 1021
Number of significant figures = 2
Subscribe to:
Comments (Atom)